|
|
|
|
 |
OTHER MAMMALS: Bats |
|
|
Fig. 1. Little brown bat,
Myotis lucifugus
Introduction
Conservation and Public
Education
Despite their ecological
value, bats are relentlessly and unjustifiably
persecuted. Bats are often killed because they live near
people who needlessly fear them. These actions emphasize
the need to educate the public on the reasons for bat
conservation and why it is important to use safe,
nondestructive methods to alleviate conflicts between
people and bats. General sources of information on bats
include states’ Cooperative Extension Services,
universities, government environmental conservation and
health departments, and Bat Conservation International
(Austin, Texas). Except where control is necessary, bats
should be appreciated from a distance — and not
disturbed.
Identification and Range
Bats, the only mammals
that truly fly, belong to the order Chiroptera. Their
ability to fly, their secretiveness, and their nocturnal
habits have contributed to bat folklore, superstition,
and fear. They are worldwide in distribution and include
about 900 species, second in number only to Rodentia
(the rodents) among the mammals.
Among the 40 species of
bats found north of Mexico, only a few cause problems
for humans (note that vampire bats are not found in the
United States and Canada). Bats congregating in groups
are called colonial bats; those that live a lone
existence are known as solitary bats.
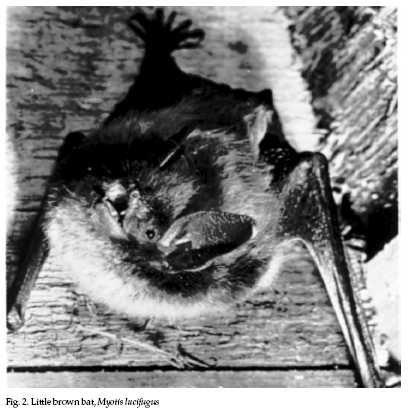
The colonial species most
often encountered in and around human buildings in the
United States are the little brown bat, (Myotis
lucifugus, Fig. 2), the big brown bat (Eptesicus fuscus,
Fig. 3), the Mexican free-tailed bat (Tadarida
brasiliensis, Fig. 4), the pallid bat (Antrozous
pallidus), the Yuma myotis (Myotis yumanensis), and the
evening bat (Nycticeius humeralis).
Solitary bats typically
roost in tree foliage or under bark, but occasionally
are found associated with buildings, some only as
transients during migration.

These include Keen’s bat (Myotis
keenii), the red bat (Lasiurus borealis), the
silver-haired bat (Lasionycteris noctivagans), and the
hoary bat (Lasiurus cinereus). Excellent illustrations
of all bats discussed herein can be found in Barbour and
Davis (1979), Tuttle (1988), Geluso et al. (1987), and
Harvey (1986).
Several species of bats
have been included here, with significant interspecific
differences that need to be clarified if well-planned,
comprehensive management strategies are to be developed.
Any problems caused by bats are limited to species
distribution; thus animal damage control personnel need
not be concerned with every species.
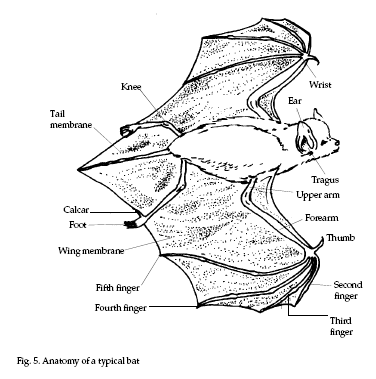
Colonial and solitary bats
have obvious differences that serve to separate the
species into groups (refer to Fig. 5). Much of the
descriptive material that follows is adapted from
Barbour and Davis (1979).
Distribution Maps for various North American Bats
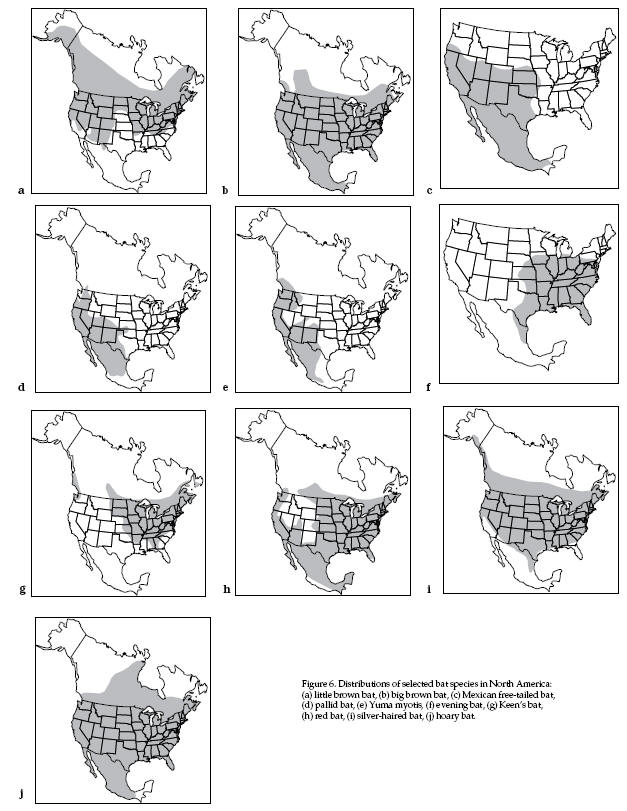
Colonial Bats
Little brown bat (Myotis
lucifugus)
Recognition
forearm — 1.34 to 1.61
inches (3.4 to 4.1 cm)
wingspan — 9.02 to 10.59
inches (22.9 to 26.9 cm)
ears — 0.55 to 0.63 inches
(1.4 to 1.6 cm)
foot — approximately 0.39
inches (1.0 cm); long hairs on toes extend beyond claws.
Distribution
(Fig. 6a)
Color
Pale tan through reddish
brown to dark brown, depending on geographic location.
The species is a rich dark brown in the eastern United
States and most of the west coast. Fur is glossy and
sleek.
Confusion may occur with a
few other “house” bat species. In the East, it may be
confused with Keen’s bat (M. keenii), which has longer
ears [0.69 to 0.75 inches (1.7 to 1.9 cm)] and a longer,
more pointed tragus (the appendage at the base of the
ear). In the West, it resembles the Yuma myotis (M.
yumanensis), which has dull fur and is usually smaller.
However, the Yuma myotis and little brown may be
indistinguishable in some parts of the northwestern
United States where they may hybridize.
Habits
This is one of the most
common bats found in and near buildings, often located
near a body of water where they forage for insect prey.
Summer colonies are very gregarious, commonly roosting
in dark, hot attics and associated roof spaces where
maternity colonies may include hundreds to a few
thousand individuals. Colonies may also form beneath
shingles and siding, in tree hollows, beneath bridges,
and in caves. Litter size is 1 in the Northeast; twins
occasionally occur in some other areas. The roost is
often shared with the big brown bat (E. fuscus) though
the latter is less tolerant of high temperatures; M.
keenii may also share the same site. Separate groups of
males tend to be smaller and choose cooler roosts within
attics, behind shutters, under tree bark, in rock
crevices, and within caves.
In the winter, little
brown bats in the eastern part of their range abandon
buildings to hibernate in caves and mines. Such
hibernacula may be near summer roosts or up to a few
hundred miles (km) away. Little is known of the winter
habits of M. lucifugus in the western United States.
The life span of little
brown bats has been established to be as great as 31
years. The average life expectancy, however, is probably
limited to only a few years.
Big brown bat (Eptesicus
fuscus)
Recognition
forearm — 1.65 to 2.01
inches (4.2 to 5.1 cm)
wingspan — 12.80 to 13.78
inches (32.5 to 35.0 cm)
ears — with rounded tragus
Distribution (Fig. 6b)
Color
From reddish brown, copper
colored, to a dark brown depending on geographic
location. This is a large bat without distinctive
markings.
Confusion may occur with
the evening bat (Nycticeius humeralis) though the latter
is much smaller.
Habits
This hardy, rather
sedentary species appears to favor buildings for
roosting. Summer maternity colonies may include a dozen
or so and up to a few hundred individuals, roosting
behind chimneys, in enclosed eaves, in hollow walls,
attics, barns, and behind shutters and unused sliding
doors. They also form colonies in rock crevices, beneath
bridges, in hollow trees, and under loose bark. Litter
size is 2 in the East to the Great Plains; from the
Rockies westward 1 young is born.
E. fuscus frequently
shares roosts with M. lucifugus in the East, and with M.
yumanensis, Taderida, and Antrozous in the West. Males
typically roost in smaller groups or alone during the
summer.
The big brown bat is one
of the most widely distributed of bats in the United
States and is probably familiar to more people than any
other species. This is partially due to its large,
easy-to-observe size, but also to its ability to
overwinter in buildings (attics, wall spaces, and
basements). Its close proximity to humans, coupled with
its tendency to move about when temperature shifts
occur, often brings this bat into human living quarters
and basements in summer and winter. Big browns also
hibernate in caves, mines, storm sewers, burial vaults,
and other underground harborage. While E. fuscus will
apparently travel as far as 150 miles (241 km) to
hibernacula, the winter quarters of the bulk of this
species are largely unknown.
Big brown bats may live as
long as 18 years.
Mexican free-tailed bat (Tadarida
brasiliensis)
Recognition
forearm — 1.42 to 1.81
inches (3.6 to 4.6 cm)
wingspan — 11.42 to 12.80
inches (29.0 to 32.5 cm); long narrow wings
tail (interfemoral)
membrane — does not enclose the lower one-third to
one-half of the tail, hence the name free-tailed
foot — long, stiff hairs
as long as the foot protrude from the toes.
Distribution (Fig. 6c)
Color
Dark brown or dark gray.
Fur of some individuals may have been bleached to a pale
brown due to ammonia fumes from urine and decomposing
guano.
Confusion is not likely to
occur with other species that commonly inhabit human
buildings.
Habits
T. brasiliensis forms the
largest colonies of any warm-blooded animal,
establishing sizable colonies in buildings, particularly
on the West Coast and in the Gulf states from Texas
east. Hundreds to thousands may be found in buildings or
under bridges. It is primarily a cave bat in Arizona,
New Mexico, Oklahoma, and Texas; buildings are used as
temporary roosts during migrations. Litter size is 1.
Taderida often share
roosts with other species. In the West, for example,
they may be found in buildings with A. pallidus, M.
yumanensis, and E. fuscus. Some males are always present
in the large maternity colonies, but they tend to
segregate in separate caves.
A few Taderida may
overwinter in buildings as far north as South Carolina
in the East and Oregon in the West. Most of this species
migrate hundreds of miles to warmer climes (largely to
Mexico) for the winter.
Pallid bat (Antrozous
pallidus)
Recognition
forearm — 1.89 to 2.36
inches (4.8 to 6.0 cm)
wingspan — 14.17 to 15.35
inches (36.0 to 39.0 cm)
ears — large; widely
separated and more than half as broad as long. The ears
are nearly half as long as the combined length of the
bat’s head and body.
eyes — large
Distribution (Fig. 6d)
Color
pale, upper parts are
light yellow, the hairs tipped with brown or gray.
Underparts are pale creamy, almost white. This large,
light-colored bat is relatively easy to recognize.
Confusion with other
species that commonly inhabit human buildings is not
likely to occur.
Habits
Maternity colony size
ranges from about 12 to 100 individuals. Roost sites
include buildings, bridges, and rock crevices; less
frequently, tree cavities, caves, and mines. Litter size
is most commonly 2. The roost is frequently shared with
T. brasiliensis and E. fuscus in the West. While groups
of males tend to segregate during the nursery period
(sometimes in the same building), other males are found
within the maternity colony.
An interesting feature of
pallid bats is that they fly close to the ground, may
hover, and take most prey on the ground, not in flight.
Prey includes crickets, grasshoppers, beetles, and
scorpions. They will also forage among tree foliage.
Pallid bats are not known
to make long migrations, though little is known of their
winter habits.
Yuma myotis (Myotis
yumanensis)
Recognition
forearm — 1.26 to 1.50
inches (3.2 to 3.8 cm)
wingspan — about 9.25
inches (23.5 cm)
ears — 0.55 to 0.59 inches
(1.4 to 1.5 cm)
foot — 0.39 inches (1.0
cm)
Distribution (Fig. 6e)
Color
Light tan to dark brown;
underside is whitish to buffy.
Confusion may occur in the
West with M. lucifugus, though the latter tends to have
longer, glossier fur, and is larger. In the Northwest,
hybridization occurs with M. lucifugus, making the
species indistinguishable.
Habits
Maternity colonies, up to
several thousand individuals, form in the summer in
attics, belfries, under bridges, and in caves and mines.
Litter size is 1. Males typically segregate during the
nursery period and roost as solitary individuals in
buildings and other suitable harborage.
M. yumanensis is more
closely associated with water than is any other North
American bat species. Nearly all roosts have open water
nearby. This species is not as tolerant as M. lucifugus
of high roost temperatures and will move to cooler
niches within a building when temperatures rise much
above 100o F (37.8o C).
M. yumanensis abandons
maternity colonies in the fall, but its winter habitat
is not known.
Evening bat (Nycticeius
humeralis)
Recognition
forearm — 1.30 to 1.54
inches (3.3 to 3.9 cm)
wingspan — 10.24 to 11.02
inches (26.0 to 28.0 cm)
ears — with short, curved,
and rounded tragus
Confusion may occur with
the big brown bat (E. fuscus), which can be readily
distinguished by its larger size. It bears some
resemblance to the somewhat smaller little brown bat (M.
lucifugus) but can be identified by its characteristic
blunt tragus.
Distribution (Fig. 6f)
Color
Medium brown with some
variation to yellow-brown in subtropical Florida. No
distinctive markings.
Habits
Summer maternity colonies
in buildings may consist of hundreds of individuals.
Litter size is usually 2. Colonies also form in tree
cavities and under loose tree bark. In the Southeast, T.
brasiliensis commonly inhabits the same building with N.
humeralis. This is one of the most common bats in towns
throughout the southern coastal states. Very little is
known about this species, and virtually nothing is known
of its winter habitat except that it almost never enters
caves.
Solitary Bats Keen’s bat (Myotis
keenii)
Recognition
forearm — 1.26 to 1.54
inches (3.2 to 3.9 cm)
wingspan — 8.98 to 10.16
inches (22.8 to 25.8 cm)
ears — 0.67 to 0.75 inches
(1.7 to 1.9 cm); with a long, narrow, pointed tragus
Distribution (Fig. 6g)
Color
Brown, but not glossy;
somewhat paler in the East.
Confusion may occur with
M. lucifugus, which has glossy fur, shorter ears, and
does not have the long, pointed tragus.
Habits
Excluding small maternity
colonies (up to 30 individuals are on record), M. keenii
are generally found singly in the East. Roosting sites
include: behind shutters, under wooden shingles,
sheltered entryways of buildings, in roof spaces, in
barns, and beneath tree bark. In the West, this bat is
known as a solitary species, roosting in tree cavities
and cliff crevices. Litter size is probably 1. The roost
is sometimes shared with M. lucifugus. The sexes
probably segregate during the nursery period. In winter,
these bats hibernate in caves and mines.
Red Bat (Lasiurus
borealis)
Recognition
forearm — 1.38 to 1.77
inches (3.5 to 4.5 cm)
wingspan — 11.42 to 13.07
inches (29.0 to 33.2 cm); long, pointed wings
ears — short rounded
tail membrane — heavily
furred on upper surface, with a distinctive long tail.
Distribution (Fig. 6h)
Color
Bright orange to
yellow-brown; usually with a distinctive white mark on
the shoulders.
Confusion may occur with
the hoary bat (L. cinereus), which is frosted-gray in
appearance and larger.
Habits
Red bats live solitary
lives, coming together only to mate and migrate. Few
people are familiar with this species. They typically
spend summer days hidden in the foliage of deciduous
trees. The number ofyoung ranges from 1 to 4, averaging
2.3.
These bats often chase
insects that are attracted to lights, such as street
lamps. It is this behavior that most likely brings them
in close proximity to people.
L. borealis is
well-adapted for surviving drastic temperature
fluctuations; it does not hibernate in caves, but
apparently in trees. Some migrate long distances. During
migration, red bats have been known to land on high-rise
buildings and on ships at sea.
Silver-haired bat (Lasionycteris
noctivagans)
Recognition
forearm — 1.46 to 1.73
inches (3.7 to 4.4 cm)
wingspan — 10.63 to 12.20
inches (27.0 to 31.0 cm)
ears — short, rounded,
hairless
tail membrane — upper
surface is sparsely furred on the anterior one-half.
Distribution (Fig. 6i)
Usually black with
silver-tipped fur; some individuals with dark brown,
yellowish-tipped fur.
Confusion sometimes occurs
with the larger hoary bat (Lasiurus cinereus), which has
patches of hair on the ears and wings, heavy fur on the
entire upper surface of the tail membrane, and has a
distinctive throat “collar.”
Habits
The silver-haired bat
roosts in a wide variety of harborages. A typical roost
might be behind loose tree bark; other sites include
tree hollows and bird nests. This species is solitary
except when with young. Additionally, there are
unconfirmed reports that it is sometimes colonial (Dalquest
and Walton 1970) and may roost in and on buildings. The
litter size is 2. The sexes segregate through much of
the summer range.
L. noctivagans hibernates
in tree crevices, under loose bark, in buildings
(including churches, sky scrapers, and wharf houses),
hulls of ships, rock crevices, silica mines, and
non-limestone caves. It also may migrate, during which
time it is encountered in buildings (they favor open
sheds, garages, and outbuildings rather than enclosed
attics), in lumber piles, and on ships at sea.
Hoary bat (Lasiurus
cinereus)
Recognition
forearm — 1.81 to 2.28
inches (4.6 to 5.8 cm)
wingspan — 14.96 to 16.14
inches (38.0 to 41.0 cm)
ears — relatively short,
rounded, edged with black, and with fur
tail membrane — completely
furred on upper surface
Distribution (Fig. 6j)
Color
Dark, but many hairs are
tipped in white, giving it a frosted appearance. This
bat also has a yellowish or orangish throat “collar.”
Confusion may sometimes
occur with the much smaller silver-haired bat (Lasionycteris
noctivagans), which lacks the fur patches and markings
on the ears, markings on the throat, and has a tail
membrane that is only lightly furred on the upper
surface.
Habits
Hoary bats generally spend
summer days concealed in tree foliage (often in
evergreens), rarely enter houses, and are not commonly
encountered by people. L. cinereus at their day roosts
are usually solitary except when with young. The litter
size is 2. The sexes segregate through most of the
summer range.
This is one of the largest
bats in North America, a powerful flier, and an
accomplished migrant. Records indicate that some L.
cinereus may hibernate in northern parts of their range.
Food Habits
Bats in North America are
virtually all insectivorous, feeding on a variety of
flying insects (exceptions among house bats were noted
previously). Many of the insects are harmful to humans.
While there must be some limitations based on such
factors as bats’ body size, flight capabilities, and jaw
opening, insectivorous bats apparently consume a wide
range of prey (Barbour and Davis 1979). The little brown
bat’s diet includes mayflies, midges, mosquitoes, caddis
flies, moths, and beetles. It can consume insects equal
to one-third of its body weight in 1/2 hour of foraging.
The big brown bat may fill its stomach in about 1 hour
(roughly 0.1 ounce per hour [2.7 g/hr]) with prey
including beetles, moths, flying ants, true bugs,
mayflies, caddis flies, and other insects. The nightly
consumption of insects by a colony of bats can be
extremely large.
General Biology, Reproduction, and Behavior
Most North American bats
emit high frequency sounds (ultrasound) inaudible to
humans and similar to sonar, in order to avoid
obstacles, locate and capture insect prey, and to
communicate. Bats also emit audible sounds that may be
used for communication between them.
Bats generally mate in the
fall and winter, but the female retains the sperm in the
uterus until spring, when ovulation and fertilization
take place. Pregnant females may congregate in maternity
colonies in buildings, behind chimneys, beneath bridges,
in tree hollows, caves, mines, or other dark retreats.
No nests are built. Births typically occur from May
through July. Young bats grow rapidly and are able to
fly within 3 weeks. Weaning occurs in July and August,
after which the nursery colonies disperse.
Bats prepare for winter
around the time of the first frost. Some species migrate
relatively short distances, whereas certain populations
of the Mexican free-tailed bat may migrate up to 1,000
miles (1,600 km). Bats in the northern United States and
Canada may hibernate from September through May.
Hibernation for the same species in the southern part of
their range may be shorter or even sporadic. Some may
fly during warm winter spells (as big brown bats may in
the northeastern part of the United States). Bats often
live more than 10 years.
In response to a variety
of human activities, direct and indirect, several bat
species in the United States have declined in number
during the past few decades. Chemical pesticides
(particularly the use of persistent and bioaccumulating
organic pesticides) have decreased the insect supply,
and contaminated insects ingested by bats have reduced
bat populations. Many bats die when people disturb
summer maternity roosts and winter hibernacula. Vandals
and other irresponsible individuals may deliberately
kill bats in caves and other roosts. Even the activities
of speleologists or biologists may unintentionally
disturb hibernating bats, which depletes fat reserves
needed for hibernation.
Modification and
destruction of roost sites has also decreased bat
numbers. Sealing and flooding of mineshafts and caves
and general quarrying operations may inadvertently ruin
bat harborages. Forestry practices have reduced the
number of hollow trees available. Some of the
elimination of natural bat habitat may contribute to
bats roosting in buildings.
Damage and
Damage Identification
Bat Presence
Bats often fly about
swimming pools, from which they drink or catch insects.
White light (with an ultraviolet component), commonly
used for porch lights, building illumination, street and
parking-lot lights, may attract flying insects, which in
turn attract bats. Unfortunately, the mere presence of a
bat outdoors is sometimes beyond the tolerance of some
uninformed people. Information is a good remedy for such
situations.
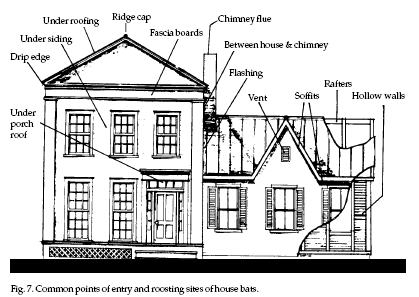 Bats
commonly enter buildings through openings associated
with the roof edge and valleys, eaves, apex of the
gable, chimney, attic or roof vent, dormers, and siding
(see Fig. 7). Bats
commonly enter buildings through openings associated
with the roof edge and valleys, eaves, apex of the
gable, chimney, attic or roof vent, dormers, and siding
(see Fig. 7).
Other openings may be
found under loose-fitting doors, around windows, gaps
around various conduits (wiring, plumbing, air
conditioning) that pass through walls, and through
utility vents.
Bats are able to squeeze
through narrow slits and cracks. For purposes of bat
management, one should pay attention to any gap of
approximately 1/4 x 1 1/2 inches (0.6 x 3.8 cm) or a
hole 5/8 x 7/8 inch (1.6 x 2.2 cm). Such openings must
be considered potential entries for at least the smaller
species, such as the little brown bat. The smaller
species require an opening no wider than 3/8 inch (0.95
cm), that is, a hole the diameter of a US 10-cent coin (Greenhall
1982). Openings of these dimensions are not uncommon in
older wood frame structures where boards have shrunk,
warped, or otherwise become loosened.
The discovery of one or
two bats in a house is a frequent problem. In the
Northeast, big brown bats probably account for most
sudden appearances (see Figs. 3 and 8). Common in urban
areas, they often enter homes through open windows or
unscreened fireplaces. If unused chimneys are selected
for summer roosts, bats may fall or crawl through the
open damper into the house. Sometimes bats may appear in
a room, then disappear by crawling under a door to
another room, hallway, or closet. They may also
disappear behind curtains, wall hangings, bookcases,
under beds, into waste baskets, and so forth. Locating
and removing individual bats from living quarters can be
laborious but is important. If all else fails, wait
until dusk when the bat may appear once again as it
attempts to find an exit. Since big brown bats may
hibernate in the cooler recesses of heated buildings,
they may suddenly appear (flying indoors or outdoors) in
midwinter during a warm spell or a cold snap as they
move about to adjust to the temperature shift.
Roosting Sites
Bats use roosting niches
that are indoors (human dwellings, outbuildings,
livestock quarters, warehouses), semi-enclosed (loading
docks, entrance foyers), partially sheltered (porches,
carports, pavilions, highway underpasses, bridges), and
open structural areas (window shutters, signs). Once
there, active bats in and on buildings can have several
economic and aesthetic effects, often intertwined with
public health issues (Frantz, 1988). Unusual roosting
areas include wells, sewers, and graveyard crypts.
Before considering control measures, verify that bats
are actually the cause of the problem.
Rub Marks
Surface areas on walls,
under loose woodwork, between bricks and around other
bat entryways often have a smooth, polished appearance.
The stained area is slightly sticky, may contain a few
bat hairs, and is yellow-brown to blackish brown in
color. The smooth gloss of these rub marks is due to
oils from fur and other bodily secretions mixed with
dust, deposited there as many animals pass repeatedly
for a long period over the same surface. Openings marked
in this way have been used heavily by bats.
Noise
Disturbing sounds may be
heard from vocalizations and grooming, scratching,
crawling, or climbing in attics, under eaves, behind
walls, and between floors. Bats become particularly
noisy on hot days in attics, before leaving the roost at
dusk, and upon returning at dawn. Note that rustling
sounds in chimneys may be caused by birds or raccoons
and scratching and thumping sounds in attics and behind
walls may indicate rats, mice, or squirrels.
Guano and Urine
Fecal pellets indicate the
presence of animals and are found on attic floors, in
wall recesses, and outside the house at its base. Fecal
pellets along and inside walls may indicate the presence
of mice, rats, or even roaches. Since most house bats
north of Mexico are insectivorous, their droppings are
easily distinguished from those of small rodents. Bat
droppings tend to be segmented, elongated, and friable.
When crushed, they become powdery and reveal shiny bits
of undigested insect remains. In contrast, mice and rat
droppings tend to taper, are unsegmented, are harder and
more fibrous, and do not become powdery when crushed
(unless extremely aged).
The droppings of some
birds and lizards may occasionally be found along with
those of bats. However, bat droppings never contain the
white chalky material characteristic of the feces of
these other animals.
Bat excrement produces an
unpleasant odor as it decomposes in attics, wall spaces,
and other voids. The pungent, musty, acrid odor can
often be detected from outside a building containing a
large or long-term colony. Similar odor problems occur
when animals die in inaccessible locations. The odor
also attracts arthropods which may later invade other
areas of a building.
Bat guano may provide a
growth medium for microorganisms, some of which are
pathogenic (histoplasmosis, for example) to humans.
Guano accumulations may fill spaces between walls,
floors, and ceilings. It may create a safety hazard on
floors, steps, and ladders, and may even collapse
ceilings. Accumulations also result in the staining of
ceilings, soffits, and siding, producing unsightly and
unsanitary conditions.
Bats also urinate and
defecate in flight, causing multiple spotting and
staining on sides of buildings, windows, patio
furniture, automobiles, and other objects at and near
entry/exit holes or beneath roosts. Bat excrement may
also contaminate stored food, commercial products, and
work surfaces.
Bat urine readily
crystallizes at room temperature. In warm conditions
under roofs exposed to sun and on chimney walls, the
urine evaporates so quickly that it crystallizes in
great accumulations. Boards and beams saturated with
urine acquire a whitish powderlike coating. With large
numbers of bats, thick and hard stalactites and
stalagmites of crystallized bat urine are occasionally
formed.
Although the fresh urine
of a single bat is relatively odorless, that of any
moderate-sized colony is obvious, and the odor increases
during damp weather. Over a long period of time urine
may cause mild wood deterioration (Frantz and Trimarchi
1984). As the urine saturates the surfaces of dry wood
beams and crystallizes, the wood fibers expand and
separate. These fibers then are torn loose by the bats
crawling over such surfaces, resulting in wood fibers
being mixed with guano accumulations underneath.
The close proximity of bat
roosts to human living quarters can result in excreta,
animal dander, fragments of arthropods, and various
microorganisms entering air ducts as well as falling
onto the unfortunate residents below. Such contaminants
can result in airborne particles of public health
significance (Frantz 1988).
Ectoparasites and other
Arthropods Several arthropods (fungivores, detritivores,
predators, and bat ectoparasites) are often associated
with colonies of bats in buildings. Their diversity
depends on the number of bats, age and quantity of
excreta deposits, and season. Arthropods such as
dermestid beetles (Attagenus megatoma) contribute to the
decomposition of guano and insect remnants, but may also
become a pest of stored goods and/or a nuisance within
the living quarters. Cockroaches (for example, Blatta
orientalis) attracted to guano may invade other parts of
a building. Bat bugs (Cimex spp.) are sometimes found
crawling on the surface of beams or around holes leading
to secluded recesses used by bats. Bat ectoparasites
(ticks, mites, fleas, and bugs) rarely attack humans or
pets and quickly die in the absence of bats.
Ectoparasites may become a nuisance, however, following
exclusion of large numbers of bats from a
well-established roost site. Area fumigation with a
total release pyrethrum based aerosol may be an
appropriate solution for arthropod knockdown within an
enclosed space, but only after bats have departed. For
long-term arthropod control, lightly dust appropriate
surfaces (affected attic beams, soffits) with boric acid
powder or diatomaceous earth; carefully read all product
labels before using any pesticide. Note that neither
rabies nor Lyme disease is transmitted by any arthropods
associated with bats.
Public Health Issues
Rabies—General
Epidemiology Bats are distinct from most vertebrate
pests that inhabit human dwellings because of the
potential for transmitting rabies — a viral infection of
mammals that is usually transmitted via the bite of an
infected animal. Rabies does not respond to antibiotic
therapy and is nearly always fatal once symptoms occur.
However, because of the long incubation period (from 2
weeks to many months), prompt vaccination following
exposure can prevent the disease in humans. Dogs, cats,
and livestock also can be protected by periodic
vaccinations. Bats are not asymptomatic carriers of
rabies. After an incubation period of 2 weeks to 6
months, they become ill with the disease for as long as
10 days. During this latter period, a rabid bat’s
behavior is generally not normal—it may be found active
during the daytime or on the ground incapable of flying.
Most human exposures are the result of accidental or
careless handling of grounded bats. Even less
frequently, bats in this stage of illness may be
involved in unprovoked attacks on people or pets (Brass,
pers. commun.; Trimarchi et al. 1979). It is during this
stage that the rabid bat is capable of transmitting the
disease by biting another mammal. As the disease
progresses the bat becomes increasingly paralyzed and
dies as a result of the infection. The virus in the
carcass is reported to remain infectious until
decomposition is well advanced.
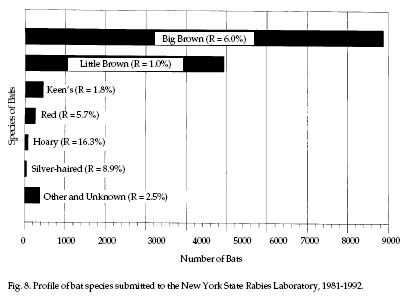 Significance.
Rabies is the most important public health hazard
associated with bats. Infection with rabies has been
confirmed in all 40 North American species of bats that
have been adequately sampled in all of the contiguous
United States and in most provinces of Canada. Figure 8
shows the frequency of bat species submitted for rabies
testing in New York State over the last 12 years. While
not a nationwide measure of human encounters with bats,
Figure 8 illustrates that bat species are not
encountered equally. Note that bats submitted for
testing are often ill and/or easily captured. The
numbers and species encountered will vary with the
region of the country; data are generally available from
local and state health authorities. Significance.
Rabies is the most important public health hazard
associated with bats. Infection with rabies has been
confirmed in all 40 North American species of bats that
have been adequately sampled in all of the contiguous
United States and in most provinces of Canada. Figure 8
shows the frequency of bat species submitted for rabies
testing in New York State over the last 12 years. While
not a nationwide measure of human encounters with bats,
Figure 8 illustrates that bat species are not
encountered equally. Note that bats submitted for
testing are often ill and/or easily captured. The
numbers and species encountered will vary with the
region of the country; data are generally available from
local and state health authorities.
Random sampling of bats
(healthy and ill) indicates an overall infection rate of
less than 1%. Finding a rabid bat in a colony does not
imply that the remaining animals are rabid. In fact, the
probability of immediately finding more than one
additional infected bat in that colony is small.
Bats rank third (behind
raccoons and skunks) in incidence of wildlife rabies in
the United States (Krebs et al. 1992). In the last 20
years, however, there have been more human rabies cases
of bat origin in the United States than of any other
wildlife group. Furthermore, the disease in bats is more
widely distributed (in all 48 contiguous states in 1989)
than in any other species. In Canada, bats also rank
third (behind foxes and skunks) in the incidence of
wildlife rabies. Therefore, every bat bite or contact
must be considered a potential exposure to rabies. While
aerosol transmission of the rabies virus from bats in
caves to humans and some other mammals has been
reported, this is not a likely route of infection for
humans entering bat roosts in buildings in temperate
North America. Note that vampire bats are not a threat
north of Mexico.
Histoplasmosis—General
Epidemiology. Histoplasmosis is a very common lung
disease of worldwide distribution caused by a
microscopic fungus, Histoplasma capsulatum. Histoplasma
exists in nature as a saprophytic mold that grows in
soil with high nitrogen content, generally associated
with the guano and debris of birds (particularly
starlings, Sturnus vulgaris, and chickens) and bats.
Wind is probably the main agent of dispersal, but the
fungus can survive and be transmitted from one site to
another in the intestinal contents of bats, and also in
the dermal appendages of both bats and birds. The
disease can be acquired by the casual inhalation of
windblown spores, but infections are more likely to
result from visits to point sources of growth of the
fungus. Relative to bats, such sources include bat
roosts in caves, barns, attics, and belfries, and soil
enriched with bat guano.
Numerous wild and domestic
animals are susceptible to histoplasmosis, but bats (and
perhaps the armadillo) are the only important animal
vectors. Unlike bats, birds do not appear to become
infected with the fungus. Both the presence of guano and
particular environmental conditions are necessary for H.
capsulatum to proliferate. In avian habitats, the
organism apparently grows best where the guano is in
large deposits, rotting and mixed with soil rather than
in nests or in fresh deposits. Specific requirements
regarding bats have not been described, though bat
roosts with long-term infestation are often mentioned in
the literature.
While histoplasmosis in
the United States is particularly endemic to the
Ohio-Mississippi Valley region (which is also an area
with the greatest starling concentration) and areas
along the Appalachian Mountains, it is also found in the
lake and river valleys of other states. Outside areas
with “appropriate” environmental conditions, there also
occur scattered foci with high infection rates usually
associated with caves inhabited by bats or birds.
Significance. When soil or
guano containing H. capsulatum is physically disturbed,
the spores become airborne. Persons at particular risk
of histoplasmosis of bat origin include spelunkers, bat
biologists, pest control technicians, people who clean
out or work in areas where bats have habitually roosted,
and people in contact with guano-enriched soil — such as
around the foundation of a building where guano has
sifted down through the walls.
Infection occurs upon
inhalation of spores and can result in a variety of
clinical manifestations; severity partially depends on
the quantity of spores inhaled. The infection may remain
localized in the lungs where it may resolve
uneventfully; this is the case for about 95% of the
500,000 infections occurring annually in the United
States. Such infections are identified only by the
presence of a positive histoplasmin skin test and/or
calcified lesions on routine radiographs. Other
individuals may have chronic or progressive lung disease
requiring treatment. Less severe forms of these
infections may be accompanied by fever, cough, and
generalized symptoms similar to a prolonged influenza.
Resolution of the disease confers a degree of immunity
to reinfection. In addition, resolution confers varying
degrees of hypersensitivity to H. capsulatum; as a
consequence, massive reinfection in highly sensitized
lungs may result in a fatal acute allergic reaction.
In a small percentage of
chronic histoplasmosis cases, the fungus disseminates to
involve multiple organ systems and may be fatal. This
form is usually seen in young children (1 year or older)
and in immunocompromised adults. In recent years,
systemic infections have been increasing in frequency
globally as an opportunistic infection of AIDS patients.
Legal Status
The lethal control of
bats, even when there is a proven potential danger to
humans, often is subjected to careful scrutiny and
interagency coordination. A survey of federal
legislative actions, court decisions, and agency
interpretations concerning bats can be found in Bat
Management in the United States (Lera and Fortune 1979).
Some states have laws that
specifically mention bats, either providing or denying
protection. Others have legislation that applies to bats
only by interpretation, since bats may be considered
nongame wildlife or indigenous state mammals. Some bats
have protection as either federal or state-listed
endangered species, but the same state may not protect
other species of bats. Enforcement and public education
must accompany legislation to accomplish the intended
goal of protecting the public and saving endangered
bats. Familiarity with the appropriate federal and state
laws should precede any nuisance management activities.
Damage Prevention and Control Methods
Pre-management
Considerations
Bat Watch for Infestation
Confirmation. To confirm that bats are actually roosting
in or on a building, look for bats flying in and out of
a site and/ or for signs of infestation. A bat watch can
be conducted by two people (more may be necessary to
observe large or complex sites) posted at opposite
corners of a structure. An evening watch begins about 30
minutes before dark and a morning watch begins about 1
hour before dawn. Observations should continue for
approximately 1 hour.
Such observations can
indicate exit/ entry points and the number of bats. With
practice, distinguishing some bat species may also be
possible. For example, compared to the big brown bat,
the little brown bat is noticeably smaller in size, and
its flight has more rapid wing beats, and more rapid
turning and darting.
It may be necessary to
watch for more than one night to compensate for weather
conditions, bats’ sensitivity to observers, noisy or
inexperienced observers, and improper use of light.
Observations can be enhanced with a standard flashlight,
but be certain to keep the bright part of the beam as
far as possible away from the exit hole being observed.
Bright light will increase bats’ reluctance to exit and
may result in an incomplete exit of the colony. A
valuable observation aid is a powerful, rechargeable
flashlight equipped with a plastic, red pop-off filter
(similar to the Kodak Wratten 89B). Also, an electric
headlamp, supplied with rechargeable batteries and
fitted to a climbing or spelunking helmet, allows
hands-off illumination outdoors as well as indoors when
exploring roost locations. Bats are sensitive to light
intensity and can visually discriminate shapes and
patterns in extremely low light situations. They can
only see in black and white; hence, the low-contrast
illumination and soft shadows produced by red light has
little effect on bats.
Locating the Roost(s). It
is not always possible or convenient to conduct a bat
watch. Thus, a detailed inspection inside the building
for bats or bat sign may be necessary to find specific
roosts. Daytime is best, especially during the warmer
part of the day. Bats roost in the most varied kinds of
buildings and in every part from cellar to attic. Some
types of buildings appear preferable (older houses,
churches, barns, proximity to water) as do certain roost
locations therein, especially areas with little
disturbance, low illumination, little air circulation,
and high temperatures. Often it is easy to locate bats,
especially in warm weather in attics or lofts, where
they may hang in clusters or side-by-side from the
sloping roof lath, beams, and so forth. However, bats
have the ability to find crevices and cavities, and if
disturbed may rapidly disappear into the angles between
converging beams, behind such beams or wallboards, into
mortise holes on the underside of beams, and into the
multilayered wall and roof fabrications. If bats cannot
be openly observed, usually there are various interior
and exterior signs of their presence. Often there are
multiple roost sites within or on a single building.
Problem Assessment. Once
it has been confirmed that bats are present, one must
determine if there is damage, if there is a health risk,
and if some intervention is warranted. There are
circumstances in which “no action” is the correct action
because of the beneficial role of bats. In cases where
there is risk of contact, damage from excreta
accumulations, stains, and so on, intervention may be
necessary.
Timing. With the exception
of disease treatment and removal of the occasional bat
intruder, timing becomes an important planning
consideration. Management procedures must not complicate
an already existing problem and should emphasize bat
conservation. Therefore, all interventions should be
initiated before the young are born or after they are
weaned and able to fly. Thus, the annual opportunity
extends from about mid-August to mid-May for much of
North America. Treatments might otherwise result in the
unnecessary death of animals (especially young unable to
fly) trapped inside, offensive odors, and attraction of
arthropod scavengers.
Disease Considerations
Rabies — Preventive
Measures. It should be noted that newspapers,
television, and other mass media sometimes misrepresent
the role of rabid bats as a risk to humans. However, the
unfortunate recent (1983 to 1993) deaths of a
22-year-old man in Texas, a 30-year-old bat scientist in
Finland, a university student in British Columbia, a
5-year-old girl in Michigan, a man in Arkansas, an
11-year-old girl in New York, and a woman in Georgia
amply underscore the need to pay prompt attention to bat
bites and other exposures.
Many rabies exposures
could be avoided if people simply refrained from
handling bats. Adults and children should be strongly
cautioned never to touch bats with bare hands. All
necessary measures should be taken to ensure that bats
cannot enter living quarters in houses and apartments.
Pet cats and dogs should be kept up-to-date in rabies
vaccinations. This is also true for pets confined
indoors, because contact with bats frequently occurs
indoors. Valuable livestock also should be vaccinated if
kept in buildings harboring bats or if in a rabies
outbreak area (NASPHV 1993). While transmission of
rabies from bats to terrestrial mammals apparently is
not common, such incidents have been reported
(Reid-Sanden et al. 1990, Trimarchi 1987). Dogs, cats,
and livestock that have been exposed to a rabid or
suspected-rabid animal, but are not currently
vaccinated, must be either quarantined or destroyed.
Lastly, pest control
technicians, nuisance wildlife control personnel,
wildlife biologists, and other individuals at particular
risk of contact with rabid bats (or other wildlife)
should receive a rabies pre-exposure vaccination. This
effective prophylaxis involves only three injections of
rabies vaccine, which are administered in the arm during
a month’s time.
Rabies — Treatment for
Exposure. If a person is bitten or scratched by a bat,
or there is any suspicion that bat saliva or nervous
tissue has contaminated an open wound or mucous
membrane, wash the affected area thoroughly with soap
and water, capture the bat without damaging the head,
and seek immediate medical attention. The incident
should be reported promptly to local health authorities
in order to arrange rabies testing of the bat.
If the bat is captured and
immediate transportation to the testing laboratory is
possible, and if immediate testing can be arranged,
postexposure treatment may be delayed several hours
until the test results are known. Postexposure
prophylaxis must be administered immediately, however,
if the bat cannot be captured, if prompt transportation
to the laboratory is not possible, if the specimen is
not suitable for reliable diagnosis, or if the test
results prove positive for rabies.
The prophylaxis has little
resemblance to that of many years ago. Today, it
consists of one dose of rabies immune globulin (human
origin) and one dose of rabies vaccine (human diploid
cell) administered preferably on the dayas known or
suspected to be contaminated with H. capsulatum should
always wear protective masks capable of filtering out
particles as small as 2 microns in diameter or use a
self-contained breathing apparatus. In areas known to be
contaminated, wear protective clothing and gloves that
can be removed at the site and placed in a plastic bag
for later decontamination via formalin and washing.
Also, clean footwear before leaving the site to prevent
spore dissemination in cars, the office, at home, and
elsewhere.
Guano deposits and
guano-enriched soils should not be unnecessarily
disturbed. Dampening with water or scheduling outdoor
work at a time when the ground is relatively wet will
minimize airborne dust. Chemically decontaminate known
infective foci with a spray of 3% formalin (see CDC
1977). To protect the environment, decontamination must
be conducted in accordance with state and local
regulations. Chemical decontamination of an “active” bat
roost should be conducted only after the bats have been
excluded or after bats have departed for hibernation.
Histoplasmosis —
Treatment.
Most infections in
normally healthy individuals are benign and self-limit-ing
and do not require specific therapy (George and Penn
1986; Rippon 1988). Treatment with an antifungal agent
may be prescribed in more severe cases; amphotericin B
and/or oral imidazole ketoconazole are typically
recommended depending on the specific nature of the
infection.
Removal of Occasional Bat Intruders
A bat that has blundered
into the living quarters of a house will usually find
its way out by detecting air movement. When no bite or
contact with people or pets has occurred, the simplest
solution for “removing” the bat is to try to confine it
to one room, then open windows and doors leading
outdoors and allow it to escape. If the bat is present
at night, the lights should be dimmed to allow the
animal to find open doors and windows; some light is
necessary if an observer is to insure that the bat finds
its way out. If bright lights are kept on, the bat may
become confused and may seek refuge behind shelving,
curtains, hanging pictures, or under furniture.
Healthy bats normally will
not attack people even when chased. Chasing a flying bat
with a folded newspaper, tennis racket, or stick will
cause the bat to take evasive action, and a bat’s flight
reversal to avoid a wall is often misinterpreted as an
attack. These flailings, often futile, will cause a bat
to seek safety wherever possible, making escape more
difficult for the bat and more frustrating for the
human.
If the bat has difficulty
escaping, it can be captured in a hand net (for example,
an insect net [Fig. 9]).
 Otherwise,
wait for it to come to rest, quickly cover it with a
coffee can or similar container, and slide a piece of
cardboard or magazine under the can to trap the bat
inside (NYSDH 1990). Take the captured bat outdoors and
release it away from populated areas, preferably after
dark. Note that reasonably thick work gloves should be
worn at all times when trying to capture a bat. Also, if
a bite or physical contact occurs, capture the bat
without damaging its head and immediately contact a
physician (see previous section regarding rabies
treatment). Management of problems involving bat
colonies require more complicated procedures and a
greater time commitment. Otherwise,
wait for it to come to rest, quickly cover it with a
coffee can or similar container, and slide a piece of
cardboard or magazine under the can to trap the bat
inside (NYSDH 1990). Take the captured bat outdoors and
release it away from populated areas, preferably after
dark. Note that reasonably thick work gloves should be
worn at all times when trying to capture a bat. Also, if
a bite or physical contact occurs, capture the bat
without damaging its head and immediately contact a
physician (see previous section regarding rabies
treatment). Management of problems involving bat
colonies require more complicated procedures and a
greater time commitment.
Exclusion
Preventive Aspects. The
most satisfactory and permanent method of managing
nuisance bats is to exclude them from buildings. Locate
bats and their points of exit/entry through bat watches
or other inspection methods. This is a tedious process
to locate all openings in use, and bats may switch to
alternate ones when normal routes become unavailable.
Thus, consider “potential” as well as “active” points of
access.
Often it is apparent where
bats might gain entrance even when such openings are not
directly observable. By standing in various locations of
a darkened attic during daylight hours, one often can
find leaks of light at the extreme parts of eaves, in
layers of subroofing, and below chimney flashings. Seal
all gaps of 1/4 x 1 1/2 inches (0.6 x 3.8 cm) and
openings 5/8 x 7/8 inch (1.6 x 2.2 cm) or greater.
Bats will also use some of
the same obscure holes in buildings through which heat
(or cooled air) is lost; thus, bat-proofing often
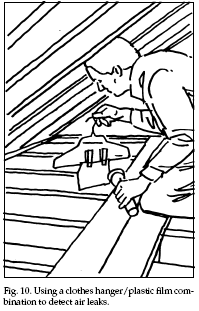
conserves energy. Simple, homemade devices can be used
to locate air leaks. Bathroom tissue or very thin
plastic film bags can be taped to a clothes hanger. When
placed in front of an area with an air leak (for
example, around window frames and sashes where caulking
or weatherstripping are needed), the tissue or plastic
will wave and flutter from air movements (Fig. 10).
Indoor air leaks can be found easily by the use of an
air flow indicator (Fig. 11). Small-volume smoke
generators can be used to locate openings in the floor,
ceiling, attic, and basement. Obscure openings also may
be located from outside the house by activating smoke
candles or smoke bombs (as within an attic), which will
produce easily observed dense smoke. Be careful of any
fire hazards.
The easiest time to seal
bats out of buildings in northern latitudes is during
the cooler part of the year when colonies are not
resident. During this period, many homeowners need to be
reminded that bats, and bat problems, return each
summer. Basic carpentry, masonry, and tinsmith skills
are valuable in bat exclusion and other pestproofing
interventions.
Devices and Methods.
Exclusion becomes “denial of reentry” once the bats have
returned to establish maternity colonies (and before the
young are born), usually from April through mid-May in
the Northeast. Denial of reentry is also appropriate
anytime after mid-August when young are capable of
flying, as long as bats continue to utilize the roost.
 The
traditional way to exclude bats from an occupied roost
involves five basic steps: (1) identify and close all
indoor openings through which bats might gain access to
human living quarters; (2) close most confirmed and all
unused potential exterior exits, leaving only a few
major openings (it’s best to complete this within 1 to 2
days); (3) at night shortly after the bats have departed
to feed, temporarily close the few remaining, major
exits; The
traditional way to exclude bats from an occupied roost
involves five basic steps: (1) identify and close all
indoor openings through which bats might gain access to
human living quarters; (2) close most confirmed and all
unused potential exterior exits, leaving only a few
major openings (it’s best to complete this within 1 to 2
days); (3) at night shortly after the bats have departed
to feed, temporarily close the few remaining, major
exits;
(4) check the roost for
presence of bats and, if any remain, unplug the
temporarily closed exits early the next evening to allow
the bats to escape, then temporarily replug the exits
(it may be necessary to repeat this step more than
once); and (5) when the bats are all out, permanently
seal the holes (Frantz and Trimarchi 1984, Greenhall
1982).
Patience and timing are
very important in this process. Much of this work can be
done during daylight hours except steps 3 and 4, which
require climbing on ladders and roofs at night,
sometimes with bats flying nearby. The danger of such
work is obvious and discouraging.
Some of these difficulties
have been overcome by use of the Constantine one-way
valvelike device which is installed in the last exit(s)
during the day, and permits bats to leave after dark but
prevents their reentry (Constantine 1982). Eventually
the valve should be removed and the hole(s) sealed.
Another device, the EX-100 Hanks Bat Excluder, consists
of a piece of nylon window screening, a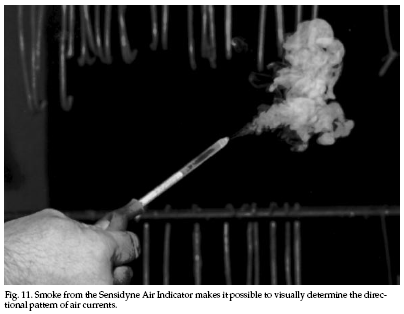 wooden plate with a hole in the middle to which is
attached a one-way plastic flappervalve, and a rigid
plastic mesh cone (Anon. 1983). The screening, to which
the wooden plate is attached, is used to cover an
opening that bats use to exit a building. Both devices
are designed to be used on the last few exit points.
Installation instructions are available, and properly
applied they will undoubtedly exclude bats from
relatively small, discrete openings.
wooden plate with a hole in the middle to which is
attached a one-way plastic flappervalve, and a rigid
plastic mesh cone (Anon. 1983). The screening, to which
the wooden plate is attached, is used to cover an
opening that bats use to exit a building. Both devices
are designed to be used on the last few exit points.
Installation instructions are available, and properly
applied they will undoubtedly exclude bats from
relatively small, discrete openings.
The devices of Constantine
and Hanks involve a one-way, self-closing valve feature
and can be readily installed during daylight hours. Such
devices are not readily adaptable to situations with
large, diffuse and/or widely distributed entryways.
Also, bats can be inadvertently trapped inside if an
important exit hole is mistakenly identified as a minor
one and is sealed in an attempt to limit the number of
holes requiring an exclusion device.
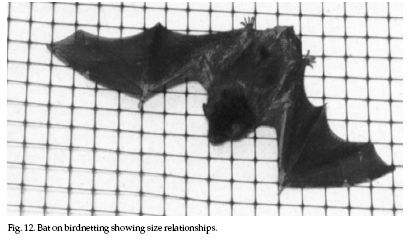 To
overcome difficulties with exclusion devices, Frantz’
checkvalve was developed using netting made of durable
black polypropylene resin (Frantz 1984, 1986). Quality
of product is important since the netting should not
fray or become misshapen under hot summer conditions.
Use only structural grade material that has openings no
larger than 1/2 x 1/2 inch (1.3 x 1.3 cm), weighs about
1.3 ounces per square yard (44 g/m2) and is flexible yet
stiff enough to maintain the shape of the checkvalve
fabricated (Fig. 12). Waterproof duct tape, common
staples, and/or wooden lath strips are used to attach
the netting to metal, slate, brick, wood, asphalt
shingle, or other surfaces. Note that duct tape may
stain or discolor painted/enameled surfaces if kept in
contact for long periods of time. To
overcome difficulties with exclusion devices, Frantz’
checkvalve was developed using netting made of durable
black polypropylene resin (Frantz 1984, 1986). Quality
of product is important since the netting should not
fray or become misshapen under hot summer conditions.
Use only structural grade material that has openings no
larger than 1/2 x 1/2 inch (1.3 x 1.3 cm), weighs about
1.3 ounces per square yard (44 g/m2) and is flexible yet
stiff enough to maintain the shape of the checkvalve
fabricated (Fig. 12). Waterproof duct tape, common
staples, and/or wooden lath strips are used to attach
the netting to metal, slate, brick, wood, asphalt
shingle, or other surfaces. Note that duct tape may
stain or discolor painted/enameled surfaces if kept in
contact for long periods of time.
Application of checkvalves
follows the same two initial steps as traditional bat
exclusion. Close interior openings, then close exterior
openings except a few major exits. These latter openings
will have been confirmed as important via bat watches,
and it is here that checkvalves will be fitted during
the daylight.
The basic design is to
attach the netting around an exit hole except at the
bottom where the bats will escape (see Frantz 1986, for
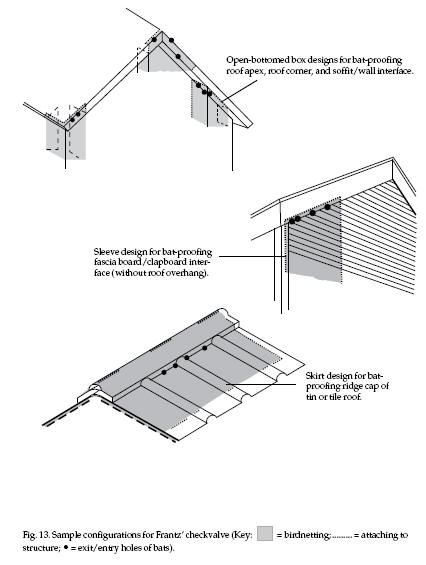
details). The width and shape of checkvalves is highly
variable so as to embrace the necessary exit point — a
single hole, a series of holes, or a long slitlike
opening (Fig. 13). Designs must be open enough not to
impede the exiting bats. The top can be much larger than
the bottom. It is probably best to restrict the bottom
opening to no larger than about 1.6 x 1.6 feet (0.5 x
0.5 m). The length of a checkvalve, that is, the
distance from the lowest enclosed point of egress to the
bottom of the netting, should be about 3.3 feet (1 m).
The above specifications
usually are sufficient to abort bats’ reentry attempts.
If netting is applied while young are still in the
roost, the “evicted” mothers may be motivated to chew
holes in the netting to reenter the roost. Applied at
the correct time of year, however, netting will allow
all bats to exit at dusk and thereafter deny them
reentry.
Checkvalves should be kept
in place for 3 to 5 days. It is best to verify (conduct
a bat watch) that bats no longer exit at dusk before the
checkvalves are dismantled and the holes are sealed
permanently. As in any exclusion intervention, the
excluded animals will go elsewhere. This shift may be to
an alternative roost already in use such as a night
roost, or one used in previous years.
 Supplemental
Materials and Methods. While specifications for Frantz’
checkvalve have been provided, additional caulking,
flashing, screening, and insulation materials often are
needed. The combination of materials used will depend on
the location, size, and number of openings, and the need
for ventilation. Greenhall (1982) provides many details
of bat-proofing methods and materials and is a practical
guide. Weatherstripping, knitted wire mesh (Guard-All®,
Stuf-fitare best applied during dry periods when wood
cracks are widest. Caulks that may be applied with a
caulking gun (in gaps up to about 0.4 inch [1 cm] wide)
include latex, butyl, and acrylic, which last about 5
years. Elastomeric caulks, such as silicone rubber, will
last indefinitely, expand and contract, do not dry or
crack, and tolerate temperature extremes. Oakum packs
easily and firmly into small cracks. Other fillers
include sponge rubber, glass fiber, knitted wire mesh,
and quick-setting putty. Self-expanding polyurethane
foam applied from pressurized containers can be used for
openings larger than 3 inches (>7.5 cm). It must be
applied with caution so as to not lift clapboards,
shingles, and other surfaces. Exposed surfaces should be
sealed with epoxy paint to prevent insect infestation
and ultraviolet degradation. Supplemental
Materials and Methods. While specifications for Frantz’
checkvalve have been provided, additional caulking,
flashing, screening, and insulation materials often are
needed. The combination of materials used will depend on
the location, size, and number of openings, and the need
for ventilation. Greenhall (1982) provides many details
of bat-proofing methods and materials and is a practical
guide. Weatherstripping, knitted wire mesh (Guard-All®,
Stuf-fitare best applied during dry periods when wood
cracks are widest. Caulks that may be applied with a
caulking gun (in gaps up to about 0.4 inch [1 cm] wide)
include latex, butyl, and acrylic, which last about 5
years. Elastomeric caulks, such as silicone rubber, will
last indefinitely, expand and contract, do not dry or
crack, and tolerate temperature extremes. Oakum packs
easily and firmly into small cracks. Other fillers
include sponge rubber, glass fiber, knitted wire mesh,
and quick-setting putty. Self-expanding polyurethane
foam applied from pressurized containers can be used for
openings larger than 3 inches (>7.5 cm). It must be
applied with caution so as to not lift clapboards,
shingles, and other surfaces. Exposed surfaces should be
sealed with epoxy paint to prevent insect infestation
and ultraviolet degradation.
Conventional draft sweeps
(metal, rubber) and other weatherstripping supplies
(felt, vinyl, metal) will seal the space between a door
bottom and the threshold or around windows (Fig. 14).
Remember to treat attic and basement doors whenever the
gap exceeds 1/4 inch (0.6 cm). Flashing may be used to
close gaps wherever joints occur; for example, where the
roof meets a chimney. Materials commonly used include
galvanized metal, copper, aluminum, and stainless steel.
Self-adhesive stainless steel “tape” is also available.
Insulation will provide some degree of barrier to bat
movements. It is available in a number of forms and
types including fiberglass, rock wool, urethane,
vermiculite, polystyrene, and extruded polystyrene foam.
Inorganic materials are fire and moisture resistant;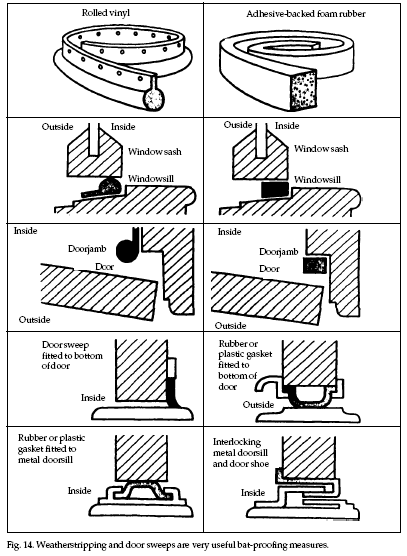 the safest appear to be fiberglass and rock wool.
the safest appear to be fiberglass and rock wool.
The mesh size of screening
must be small enough to prevent access of bats and other
species, where desired. Hardware cloth with 1/4-inch
(0.6-cm) mesh will exclude bats and mice; screening with
16 meshes per inch (2.5 cm) will exclude most insects.
Soffits (underside of overhanging eaves) usually have
ventilators of various shapes and sizes. Regardless of
type, the slots should not exceed 1/4 x 1 inch (0.6 cm x
2.5 cm) and should be covered inside with insect mesh.
To prevent bats from entering chimney flues, completely
enclose the flue discharge area with rust-resistant
spark arresters or pest screens, secured to the top of
the chimney. These should not be permanently attached
(for example, with screws) in case they must be rapidly
removed in the event of a chimney fire. Review fire
codes before installing flue covers. Dampers should be
kept closed except in the heating season.
Roof Problems. Bats,
particularly the Mexican free-tailed bat, often roost
under Spanish or concrete tile roofing by entering the
open ends at the lowermost row or where the tiles
overlap (Fig. 15). Tight-fitting plugs are difficult to
make due to the variation in opening sizes and thermal
expansion and contraction. A solution was found by
Constantine (1979) in which a layer of coarse fiberglass
batting was laid under the tiles so that bats entering
holes would contact the fiberglass and be repelled. A
layer of knitted wire mesh would undoubtedly work well
for this purpose (and would not hold moisture). Bats
also may be excluded from the tiles if rain gutters are
installed directly under the open ends. Gaps under
corrugated and galvanized roofing may be closed with
knitted wire mesh, self-expanding foam (avoid causing
roofing to lift), or with fiberglass batting (may retain
moisture). Wall Problems. Fiberglass or rock wool
insulation blown into wall spaces that are used by bats
may be a deterrent, especially when it forms a physical
barrier to passage. Such work must be done when bats are
absent to avoid their entrapment.
 Temporary
Roosts. Bats will sometimes temporarily roost on porches
and patios, in garages, and behind shutters, shingles,
and roof gutters. Roosting behind shutters may also be
long-term in duration. Actual control measures may not
be necessary unless bat droppings become a problem or
the risk of human contact is significant. Coarse
fiberglass batting tacked to the surfaces where bats
prefer to hang sometimes discourages them. A potentially
useful intervention for the wall-ceiling interface is
the application of a wide 45o molding strip to eliminate
the 90o angle corner and force the bats to roost in a
more exposed area. Temporary
Roosts. Bats will sometimes temporarily roost on porches
and patios, in garages, and behind shutters, shingles,
and roof gutters. Roosting behind shutters may also be
long-term in duration. Actual control measures may not
be necessary unless bat droppings become a problem or
the risk of human contact is significant. Coarse
fiberglass batting tacked to the surfaces where bats
prefer to hang sometimes discourages them. A potentially
useful intervention for the wall-ceiling interface is
the application of a wide 45o molding strip to eliminate
the 90o angle corner and force the bats to roost in a
more exposed area.
Repellents
While many chemical
aromatics and irritants have been proposed and tested
for bat repellency, efficacy has been very limited thus
far.
Naphthalene crystals and
flakes are the only repellents registered by the US
Environmental Protection Agency (EPA) for indoor bat
control and are to be applied in attics or between
walls. Sometimes the chemical may be placed in
loose-mesh cloth bags and suspended from the rafters.
About 2.5 pounds per 1,000 cubic feet (1.2 kg/30 m3) is
recommended to chronically repel bats as the chemical
vaporizes. Dosages of 5 pounds per 1,000 cubic feet (2.4
kg/30 m3) may dislodge bats in broad daylight. Bats will
return, however, when the odor dissipates. The prolonged
inhalation of naphthalene vapors may be hazardous to
human health.
Illumination has been
reported to be an effective repellent. Floodlights
strung through an attic to illuminate all roosting sites
may cause bats to leave. Large attics may require many
100-watt bulbs or 150-watt spotlights to be effective.
Fluorescent bulbs may also be used. In some situations
such lighting is difficult, costly, and m trical hazard.
Where possible, the addition of windows to brighten an
attic will help to reduce the desirability of the roost
site and is not likely to introduce additional problems.
Air drafts have
successfully repelled bats in areas where it is possible
to open doors, windows, or create strong breezes by use
of electric fans. Addition of wall and roof vents will
enhance this effort, as well as lower roost temperature.
These measures will increase the thermoregulatory burden
on the bats, thus making the roost less desirable. In a
similar fashion, colonies located in soffits, behind
cornices, and other closed-in areas can be discouraged
by opening these areas to eliminate dark recesses.
Discourage bats from roosting behind shutters by
removing the shutters completely or by adding small
blocks at the corners to space them a few inches away
from the wall.
Ultrasonic devices have
been tested under natural conditions, both indoors and
outdoors, to repel little brown and big brown bats
either in the roost or as they fly toward an entrance
hole (Frantz, unpublished data). The results have not
been promising. Numerous ultrasonic devices have been
removed from clients’ homes because the bats remained in
the roost after the devices were activated. Hurley and
Fenton (1980) exposed little brown bats to ultrasound in
seminatural roosts with virtually no effect. Largely
because of this lack of known scientific efficacy for
ultrasonic devices, the New York State Consumer
Protection Board has cautioned against the use of such
devices (NYSCPB 1988). Part of the concern is that such
devices will provide consumers with a false sense of
security and, thus, may prevent them from taking
effective preventive actions.
Distress cries of bats
recorded on tape and rebroadcast can be used to attract
other bats to nets or traps, but they do not serve as an
effective repellent. Little brown and big brown bats
respond to their own distress cries but not to the cries
of other species.
Contact repellents, such
as sticky-type bird repellents and rodent glues, have
been used successfully in situations where roost
surfaces and bat accesses may be coated. Apply masking
tape to the surface first if you desire to remove the
repellent after treatment is finished. Replenish contact
repellents occasionally, since dust accumulation causes
them to lose their tackiness. Also, caution must be
exercised so as to apply coatings that will be sticky,
but will not entrap the bats.
Toxicants (not
recommended)
No toxicants are registered for controlling bats. In
1987 the Centers for Disease Control, United States
Department of Health and Human Services, voluntarily
withdrew the last registration for DDT use against bats
in the United States. Thus, DDT is no longer registered
for any use in this country.
Although federally
registered for rodents, chlorophacinone (RoZol )
tracking powder, an anticoagulant, is not registered for
bats. Furthermore, it can no longer be registered by
individual states for restricted use under Section 24(c)
of the Federal Insecticide, Fungicide, and Rodenticide
Act D-18 (FIFRA). Lipha Tech, Inc. (the manufacturer of
RoZol ) has voluntarily cancelled its registration for
“RoZol Tracking Powder for Control of Nuisance Bats” —
effective December 16, 1991 (Fed. Reg., 1991).
Trapping
Kunz and Kurta (1988)
reviewed an extensive variety of efficient methods for
trapping bats from buildings and other roosting sites or
foraging areas. For purposes of wildlife damage control,
however, exclusion is less complicated to carry out,
less time-consuming, more effective, and requires no
handling of bats.
Other Methods
Sanitation and Cleanup.
Once bats have been excluded, repelled, or have departed
at the end of the summer, measures must be completed to
make reinfestation less likely, and to eliminate odor
and problematic bioaerosols. As a prelude to such work,
it is sometimes useful to apply a pyrethrum-based,
total-release aerosol insecticide to eliminate unwanted
arthropods.
The safe handling and
removal of bat guano has been discussed previously (see
the histoplasmosis section in this chapter). In addition
to the more bulky accumulations of excreta, there are
often diffuse deposits of guano under/ among insulation
materials, caked urine and guano on roof beams, and
splattered urine on windows. Such clean-up work during
hot summer weather may be the least desirable activity
of a management program, but it is necessary.
 All
caked, crystallized bat urine and droppings should be
scraped and wire-brushed, as necessary, from all roof
and attic beams. For this procedure, workers should take
the same precautions as outlined for
histoplas-mosis-related work. Accumulated excreta and
contaminated insulation should be sealed in plastic bags
and removed for disposal. Remove all remaining droppings
and debris with a vacuum cleaner, preferably one that
has a water filter to reduce the amount of dust that
escapes from the cleaner’s exhaust. All
caked, crystallized bat urine and droppings should be
scraped and wire-brushed, as necessary, from all roof
and attic beams. For this procedure, workers should take
the same precautions as outlined for
histoplas-mosis-related work. Accumulated excreta and
contaminated insulation should be sealed in plastic bags
and removed for disposal. Remove all remaining droppings
and debris with a vacuum cleaner, preferably one that
has a water filter to reduce the amount of dust that
escapes from the cleaner’s exhaust.
Where possible, wash with
soap and water all surfaces contaminated with urine and
guano. Allow the surfaces to dry, then disinfect them by
misting or swabbing on a solution of 1 part household
bleach and 20 parts tap water. Ventilate the roost site
to allow odors and moisture to escape. Installation of
tight-fitting window screens, roof and/or wall
ventilators in attics will enhance this process.
Remember, sanitation and cleanup accompanies
bat-proofing and exclusion measures, it does not replace
them.
Artificial Roosts. For
more than 60 years, artificial bat roosts have been used
in Europe. Only recently have they gained some
popularity in the United States. Though the results are
variable, it appears that artificial roosts, if properly
constructed and located, can attract bats that are
displaced or excluded from a structure. The Missouri
Department of Conservation described a successful “bat
refuge” that was quickly occupied by a displaced colony
of little brown bats (LaVal and LaVal 1980). Bat houses
of a similar design have been successfully used in
Minnesota, New York, and elsewhere (see Fig. 16).
Development of an
efficient method to relocate bats into alternative
roosts after they have been excluded from buildings
could be an important intervention in comprehensive bat
management. Frantz (1989) found it helpful to “seed”
newly constructed bat houses with several bats, a
procedure that later resulted in full-scale colonization
without further human interventions. Alternative roosts
should be located away from human high-use areas. Thus,
people can enjoy the benefits of bats without sharing
their dwellings with them and with little risk of direct
contact with them.
Economics of Damage and Control
Virtually all bats are of
some economic importance; those north of Mexico are
beneficial because of their insectivorous diet which
eliminates many insect pests of humans. The accumulated
bat droppings, called guano, is rich in nitrogen and is
a good organic fertilizer. At one time, bat guano was
commercially mined in the Southwest; but its importance
has declined due to reduced bat populations and the
development of inorganic fertilizers. Bat guano is still
considered a valuable fertilizer resource in some parts
of the world (such as Thailand and Mexico).
No figures are available
to determine the extent of damage caused by nuisance
bats or the cost for their control. The problem is
widespread in this and other countries.
Costs for remedial
services are highly variable, depending on the nature of
the problem and who will do the work. For example, to
fabricate a few Frantz’ checkvalves on the “average”
two-story house would probably require two workers about
one-half day, mostly on stepladders, and less than $50
in materials. Much more time would be required to seal
up all the other active and potential bat exit/ entry
holes. In addition, if a deteriorated roof, eaves, or
other woodwork must be replaced, the costs can increase
rapidly.
It is often difficult or
expensive for the public to obtain the services of
reliable, licensed pest control operators (PCOs). Many
PCOs have limited knowledge of basic bat biology and are
apprehensive to work with bats. They may want to avoid
any liabilities should bat-human contact occur. Select a
qualified professional service that concentrates on the
exclusion of live bats from a structure rather than on
use of lethal chemicals.
Acknowledgments
The authors wish to thank
the many people who have allowed them and other bat
researchers to work in and about their homes, and have,
thus, contributed to this effort. We give special thanks
to Roger W. Barbour and Wayne H. Davis for permission to
reproduce figures from their excellent book, Bats of
America (University Press of Kentucky). Charles V.
Trimarchi is acknowledged for carefully reviewing this
chapter and providing many useful comments. We thank
Debra VonZwehl and Christine Borecki for processing the
manuscript.
Figures 2 through 4 from
Barbour and Davis (1979).
Figure 5 adapted from
Harvey (1986).
Figure 6 adapted from
Tuttle (1988), except Yuma myotis and Keen’s bat (from
Barbour and Davis 1979).
Figure 7 adapted from
Trimarchi andFrantz (1985).
Figure 8 by R. Suss.
Figures 12, 15, and 16 by
S. C. Frantz.
Figures 9, 10, 11, and 14
from Greenhall (1982)
Figure 13 by S. C. Frantz
For Additional Information
Anonymous. 1983. Wisconsin
firm develops bat excluder. Pest. Control Technol.
11:74.
Anonymous. 1986. States
focus on bat conservation. Bats. 3(3): 3-4.
Barbour, R. W., and W. H.
Davis. 1979. Bats of America. Univ. Kentucky Press,
Lexington. 286 pp.
Barclay, R. M. R., D. W.
Thomas, and M. B. Fenton. 1980. Comparison of methods
used for controlling bats in buildings. J. Wildl.
Manage. 44: 501-506.
CDC. 1977. Histoplasmosis
control. US Dep. Health, Educ. and Welfare, Centers for
Disease Control, Atlanta, Georgia. 10 pp.
Constantine, D. G. 1979.
Bat rabies and bat management. Bull. Soc. Vector Ecol.
4:1-9.
Constantine, D. G. 1982.
Bat-proofing of buildings by installation of valve-like
devices in entryways. J. Wildl. Manage. 46:507-513.
Dalquest, W. W., and D. W.
Walton. 1970. Diurnal retreats of bats. Pages 162-187,
in B. H. Slaughter and D. W. Walton, eds. About bats.
Southern Methodist Univ. Press, Dallas, Texas.
Fed. Reg., 1991. Notices,
Federal Register/ August 28, 1991. 56(167):42615, 42620,
and 42621.
Fenton, M. B. 1983. Just
bats. Univ. Toronto Press, Toronto. 165 pp.
Frantz, S. C. 1984.
Excluding housebats with birdnetting. Bat Res. News.
25(3/4):40-41.
Frantz, S. C. 1986.
Bat-proofing structures with birdnetting checkvalves.
Proc. Vertebr. Pest Conf. 12:260-268.
Frantz, S. C. 1987.
Chlorophacinone, DDT and other pesticides for bat
control: efforts to prohibit use in New York State. Bat
Res. News. 28(3-4): 34.
Frantz, S. C. 1988.
Architecture and commensal vertebrate pest management.
Pages 228-295 in R. B. Kundsin, ed. Architectural design
and indoor microbial pollution. Oxford Univ. Press, New
York.
Frantz, S. C. 1989. Bat
houses in state parks: an experiment in New York. Bats.
7:14.
Frantz, S. C., and C. V.
Trimarchi. 1984. Bats in human dwellings: health
concerns and management. Proc. Eastern Wild. Damage
Control Conf. 1:299-308.
Geluso, K. N., J. Scott
Altenbach, and R. C. Kerbo. 1987. Bats of Carlsbad
Caverns National Park. Carlsbad Caverns Natural Hist.
Assoc., Carlsbad, New Mexico. 34 pp.
George, R. B., and R. L.
Penn. 1986. Histoplasmosis. Pages 69-85 in G. A. Sarosi
and S. F. Davies, eds. Fungal diseases of the lung.
Grune and Stratton, Inc., New York.
Greenhall, A. M. 1982.
House bat management. Resour. Publ. No. 143. US Dep.
Inter., Fish Wildl. Serv., Washington, DC. 33 pp.
Harvey, M. J. 1986.
Arkansas bats: a valuable resource. Arkansas Game Fish
Comm., Little Rock. 48 pp.
Hill, J. E., and J. D.
Smith. 1984. Bats: A natural history. Univ. Texas Press,
Austin. 243 pp.
Hurley, S., and M. B.
Fenton. 1980. Ineffectiveness of fenthion, zinc
phosphide, DDT and two ultrasonic rodent repellers for
control of populations of little brown bats (Myotis
lucifugus). Bull. Environ. Contam. Toxicol. 25:503-507.
Krebs, J. W., R. C.
Holman, U. Hines, T. W. Strine, E. J. Mandel, and J. E.
Childs. 1992. Rabies surveillance in the United States
during 1991. Special Report, J. Am. Veterin. Med. Assoc.
201 (12):1836-1848.
Kunz, T. H. 1982. Roosting
ecology of bats. Pages 1-55 in T. H. Kunz, ed. Ecology
of bats. Plenum Press, New York.
Kunz, T. H., and A. Kurta.
1988. Capture methods and holding devices. Pages 1-29 in
T.H. Kunz, ed. Ecological and behavioral methods for the
study of bats. Smithsonian Inst. Press, Washington, DC.
Laidlaw, W. J., and M. B.
Fenton. 1971. Control of nursery colony populations of
bats by artificial light. J. Ecol. Manage. 35:843-46.
LaVal, R. K., and M. L.
LaVal. 1980. Ecological studies and management of
Missouri bats with emphasis on cave-dwelling species.
Missouri Dep. Conserv. Terr. Ser. 8. 53 pp.
Lera, T. M., and S.
Fortune. 1979. Bat management in the United States.
Bull. Nat. Speleol. Soc. 41:3-9.
NASPHV. 1993. Compendium
of animal rabies control, 1993. Natl. Assoc. State Publ.
Health Vet., Inc. Texas Dep. Health, Austin. 4 pp.
NPCA. 1991. Controlling
bats. Technical Release ESPC 043233A, 11/13/91. Natl.
Pest. Control. Assoc., Dunn Loring, Virginia. 4 pp.
NYSCPB. 1988. The quest
for the pest. Consumer News (July 4, 1988), pp. 29, 30.
NYSDH. 1990. Bat rabies in
New York State. Publ. No. 3003, New York State Dep.
Health, Albany. 12 pp.
Reid-Sanden, F. L., J. G.
Dobbins, J. S. Smith, and D. B. Fishbein. 1990. Rabies
surveillance in the United States during 1989. Spec.
Rep., J. Am. Vet. Med. Assoc. 197:1571- 1583.
Rippon, J. W. 1988.
Histoplasmosis (Histoplasmosis capsulatum). Pages
381-423 in J. W. Rippon, ed. Medical mycology. W. B.
Saunders Co., New York.
Stebbings, B., and S.
Walsh. 1985. Bat boxes. Fauna and Floral Preserv. Soc.,
London. 16 pp.
Trimarchi, C. V. 1987.
Rabies transmission from bats to terrestrial mammals:
evidence of frequency and significance. Bat Res. News.
28(3-4):38.
Trimarchi, C. V., M. K.
Abelseth, and R. J. Rudd. 1979. Aggressive behavior in
rabid big brown bats (Eptesicus fuscus). Pages 34-35 in
Rabies Information Exchange, No. 1. US Dep. Human Health
Services, Centers for Disease Control, Atlanta, Georgia.
Trimarchi, C. V., and S.C.
Frantz. 1985. Bat control. New York State Dep. Health
Pamphlet, Albany. 6 pp.
Tuttle, M. D. 1988.
America’s neighborhood bats. Univ. Texas Press, Austin.
96 pp.
Tuttle, M. D., and D.
Stevenson. 1982. Growth and survival of bats. Pages
105-150 in T.H. Kunz, ed. Ecology of bats. Plenum Press,
New York.
US EPA. 1980. Re. Bradley
Exterminating Company, Richfield, MN, Dockett No. I.
F&R. V-604-C, May 8, 1980. US Environ. Protect. Agency,
Kansas City, Missouri. 50 pp.
Wimsatt, W. A. 1970.
Biology of bats. Vol. II. Academic Press, New York. 477
pp.
Editors
Scott E. Hygnstrom Robert
M. Timm Gary E. Larson
PREVENTION AND CONTROL OF
WILDLIFE DAMAGE — 1994
Cooperative Extension
Division Institute of Agriculture and Natural Resources
University of Nebraska -Lincoln
United States Department
of Agriculture Animal and Plant Health Inspection
Service Animal Damage Control
Great Plains Agricultural
Council Wildlife Committee
01/25/2007
Special
thanks to:
Clemson University
|